CH EMED (R4)
0.1.0 - Draft Standard for Trial Use
CH EMED (R4)
0.1.0 - Draft Standard for Trial Use
This page is part of the CH EMED (R4) (v0.1.0: DSTU 1). This is the current published version in it's permanent home (it will always be available at this URL). For a full list of available versions, see the Directory of published versions 
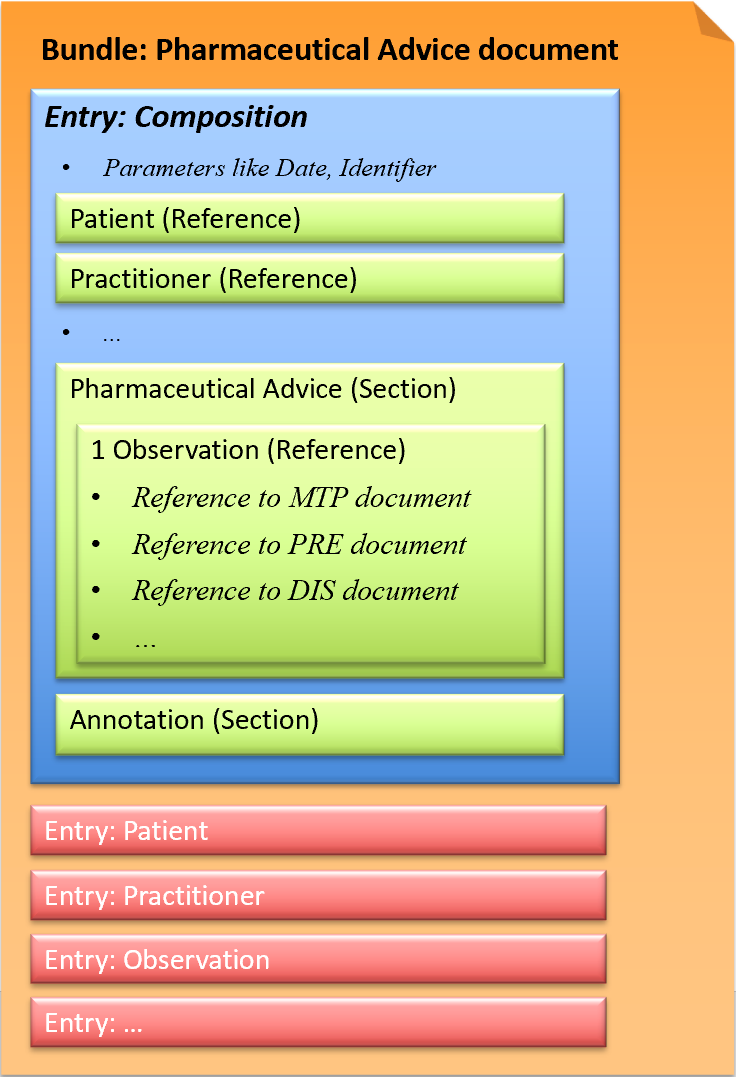
The Pharmaceutical Advice document is a document in which health professionals track important observations (with the explicit consent of the patient), in regards to medication use (ONE).
This exchange format is defined as a document type that corresponds to a Bundle as a FHIR resource. A Bundle has a list of entries. The first entry is the Composition, in which all contained entries are then referenced.
Fig.: Pharmaceutical Advice document